
But since it actually on this seesaw, you see that you'LL pull in in this direction and in that direction, and that will cancel each other out. Is the dash light indicating that's going back? So this is going to be polar because these bones here are all poor and the way that I drew it here in this Louis structure, they all perfectly cancel each other out. It looks sort of like this in face where one of these is a bond out indicated by a wedge in the other one. This is going to form a seesaw type structure. So this is f P three and then it uses a D orbital F P. You have to go higher than S P three in order to fit everything in. That's because it has six valence electrons and it's made four bonds which was a two extra electoral chances. So it's sulfur with four foreigners around it and the foreign zehr goingto have, there are tough, completely filled sulfur is going to do one of those rare exceptions where it's breaking the octet rule. But these bonds are again not gonna be one hundred eighty degrees finally with S F four. Thes here are poor bonds between the sulphur and Corinne and if it was truly when you're like this, it wouldn't be poor. They're not gonna pull evenly in each direction. So Tetra Hydro looks like sort of too wines like that and then a wedge in the dash Sort of like this on DSO This molecule is going to be poor because these bonds are going to twist it. It has left over just three low in pairs and everything here has only single bonds in it, which means it's all F p three but it's not going to be straight when here like this when you draw it because it needs to be in the hybridization of Tetra Hydro. It has seven electrons when it uses one to form a bond.
#XENON TETRAFLUORIDE ON CHEM DRAW HOW TO#
Xef6 Lewis Structure How To Draw The Lewis Structure For Xef6 Youtube. A Draw The Lewis Structures For The These Species Chegg Com. How To Draw Xef4 Lewis Structure Science Education And Tutorials. Sulfur has six valence electrons uses two for bonding, at least four leftover goingto long bears. Number Of Lone Pairs And Bonding Pairs For Xef4 Xenon Tetrafluoride Youtube. They all have the right number of bonds I need to fill in.
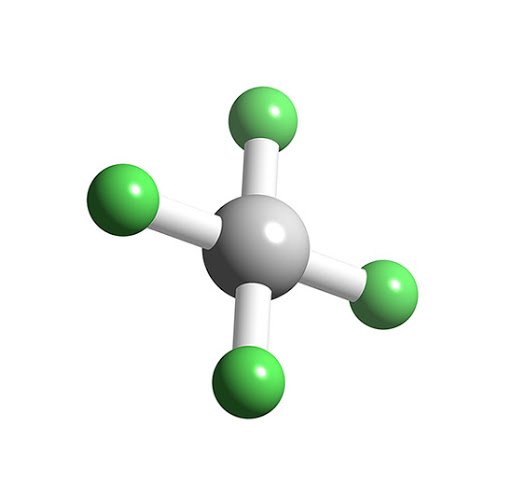
So for life tohave throughout the long pierce suffer like I have to. And this little stripped here tells us that we're goingto have a corinne bonded to its sulfur, bonded to a sulfur bonded to according the news from all you Corning liketo have one bond. They don't perfectly cancel each other out. In the boxes provided, draw the complete Lewis electron-dot diagram. But because oxygen and foreign, they're going to pull differently. (d) Xenon can react with oxygen and fluorine to form compounds such as XeO3 and XeF4. Um, and if these were all the same molecule, they would perfectly cancel each other out. I'm drawing these sort of angles indicate that they should be pointing away from each other. So these air all going to be one twenty degree angles, it's gonna fit essentially a triangle, and that will help us answer whether or not this molecules. This according to Valence bond theory, we wouldn't use thes right angles here because this is going to be tribunal, uh, Pointer. line Line art Comic book Drawing, line, comics, angle, white png gray cube logo. That's when the F P, too, and the extra pure battle that we didn't blend into this hybridization mix makes that double bond with oxygen. Osmium tetroxide Xenon tetroxide Xenon tetrafluoride Lewis structure. So since carbon has three things around it, it's going to share those into three, even Orbital's. We need Teo determined the hybridization of the interior. And since that's everything we know, it's unusual. It's got and oxygen and two florins attached to it. So for this first molecule, we know that carbon is the central Adams. So I'll just tell you guys what to do for the, um, the Vesper structure. I'm only gonna have enough room to do the Louis structures.
#XENON TETRAFLUORIDE ON CHEM DRAW PORTABLE#
So this question asked us to do a lot of things with this portable here. Journal of the American Chemical Society 1963, 85 (2), 242–242.Right. Crystal and Molecular Structure of Xenon Tetrafluoride. One of these two structures is in approximate agreement with our result. These data do not permit determination of the relative signs of the two $y$ coordinates. Ibers and Hamilton have deduced two structures by refinement of data with $h + k + l$ even. There are two single crystal-structure investigations done at room temperature, both published in 1963.


 0 kommentar(er)
0 kommentar(er)
